
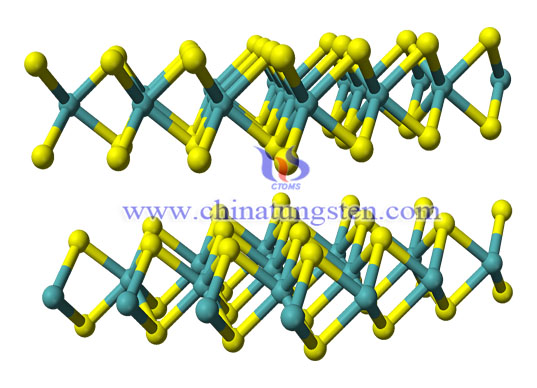
Tungsten disulfide is an inorganic chemical compound composed of tungsten and sulfur with the chemical formula WS2.
Of the compounds of sulfur and tungsten, tungsten disulfide is the only compound that can be synthesized from elements. Generally, tungsten disulfide is hexagonal crystal with the same layered structure as molybdenum disulfide. Under certain conditions, a tripartite variant of tungsten sulfide can be prepared.
Basic Information:
Name: Tungsten Disulfide
CAS: 12138-09-9
Formula:WS2
Molecular Weight:247.97
Appearance: Grey Powder
Solubility: Stable in the air, insoluble in water, alcohol, dilute nitric acid and sulfuric acid.
Pyrolysis temperature of WS2 in the air:
Stable: T≤250℃
Less Stable: 250℃< T ≤300℃
Unstable: T>300℃


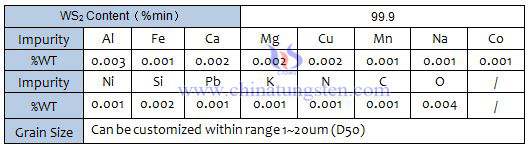


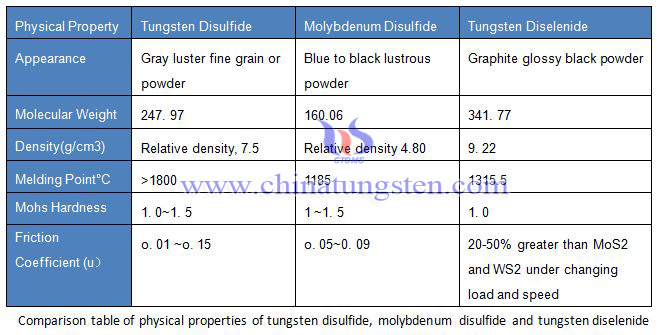
Tungsten disulfide (WS2) appears to be a gray black fine crystal powder with metallic luster, which has a greasy feeling. The relative density is 7.4~7.5, the Mohs hardness is 1.0~1.5, the compressive strength is up to 21000kg/cm2, the friction coefficient is 0.01~0.15, and it has semiconductor conductivity.
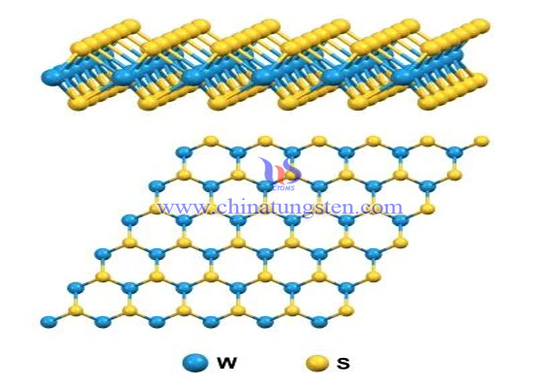
Each crystal layer of tungsten disulfide (WS2) is composed of two layers of sulfur atoms and one layer of tungsten atoms. Due to the strong combination between sulfur atoms and tungsten atoms in the crystal layer, it has high compressive capacity. The adjacent sulfur atoms of the two layers are weakly bonded, so when there is a shear force, the crystal layers will slip, showing good lubrication characteristics. Even at 1316 ° C, there is still lubrication.
Tungsten disulfide (WS2) can well adsorb on the metal surface, but does not react with the metal surface. The friction coefficient contrast test shows that the friction coefficient of tungsten disulfide is lower than that of molybdenum disulfide in the test temperature range; It is also lower than tungsten selenide at high temperature. This shows that tungsten disulfide has good high-temperature lubrication performance. The test results of friction coefficient and pressure contrast show that the friction coefficient of tungsten disulfide decreases gradually with the increase of pressure between friction pairs, and tends to a certain value. The test results of friction coefficient and air pressure show that the friction coefficient of tungsten disulfide in vacuum is low.
The thermal stability of tungsten disulfide (WS2) is not very clear. No sign of its decomposition was observed at about 1150 ° C, but it was found to be slowly sublimated in the range of 1000 to 900d ° C for example, and the sublimation product was a sound hexagonal tungsten disulfide crystal. The tungsten disulfide was heated under vacuum and began to decompose at 1200 ° C. The melting point of tungsten disulfide was above 1800 ° C. Tungsten disulfide (WS2) is melted at about 2400 ° C with high equilibrium sulfur vapor pressure.
Tungsten disulfide (WS2) is soluble in nitric acid plus hydrofluoric acid solution, insoluble in water, ethanol and oil, but can be eroded by hot sulfuric acid, hot nitric acid and hydrofluoric acid; When heated in air, it will react with oxygen to generate sulfur dioxide and tungsten trioxide; In hydrogen, tungsten and hydrogen sulfide are reduced at 800 ° C; At this time, it is stable in nitrogen.

The following are four different preparation methods of tungsten disulfide. Tungsten disulfide prepared by different methods has different crystal structures.
Method 1. Direct Synthesis
The mixture of tungsten powder and sulfur powder can be heated by nitrogen in a quartz tube and reacted at 800~900'C; This mixture can also be reacted at 110-250 ° C first, then at 550~650 ° C, and finally evaporated to remove the sulfur in the product. The latter method can obtain tungsten disulfide with purity of 99.9% and particle size of 0.1~2 µ m.
The sulfur is deposited on the tungsten contact at room temperature and heated to form tungsten disulfide; Tungsten disulfide films can be formed on various steel surfaces by ion implantation to reduce their friction coefficient and prolong their service life.
Method 2. Reaction of Tungsten and Hydrogen Sulfide
A thin layer of tungsten is deposited on the surface of quartz, placed in a furnace containing specific H2S gas, and synthesized at 1000°C. At this high temperature, some bonds of aluminum disulfide, which usually exists in the form of sheets, are broken, and the sheets curl to form a sealed cage structure.
Method 3. Preparation of WS2 from Tungsten Trioxide or Tungstic Acid
Melt the mixture of tungsten trioxide, sulfur and potassium carbonate at 900 ° C under the condition of isolating air, or mix tungstic acid, sulfur and carbon black and bake them in a reverberatory furnace; Tungsten disulfide can also be prepared by placing tungsten trioxide in flowing hydrogen sulfide and reacting at 900~1200 'C for 4 hours.
Method 4. Preparation of Tungsten Disulfide (WS2) from Iron Tetrathiotungstate
The preparation of tungsten disulfide (WS2) from iron tetrathiotungstate is a common method in industry. In this method, firstly, tungstic acid is prepared by reacting tungstic acid with ammonia, and then hydrogen sulfide is introduced to generate ammonium tetrathiotungstate, which is calcined to obtain tungsten disulfide.


Applications of WS2:
1.High-grade carbon brush wear-resistant lubricant.
2.Lubricating agents in solid lubricants.
3.For the production of self-lubricating parts and PTFE and nylon and other materials.
4.Solid anti-friction coating.
5. Due to its low friction coefficient, high compression resistance and oxidation resistance, tungsten disulfide is suitable for lubrication under high temperature, high pressure, high load and high vacuum conditions. It can be prepared into ointment, oil agent, crayon, solid self-lubricating material block and surface facial mask.
6. Tungsten disulfide can also be used as catalyst. A new type of tungsten sulfide catalyst can convert CO2 into CO for industrial raw materials.
7. The layered structure characteristics of tungsten, molybdenum and other compounds can be used to make single-layer two-dimensional materials, and can be stacked into new materials with large internal space structure as required. In addition, the required intercalation materials can be added during the stacking process, making them become catalysts or sensitive, display and superconducting materials, which has great development prospects in efficient catalysts.

Property
Film diameter: 0.02-1 μm
Number of layers: 1-10
Thickness: 1-8 nm
Stabilizer: LiOH
Application
Lubricants, catalyst, energy storage, composite materials and so on.
Store in a dry and dark sealed condition at room temperature. The maximum shelf life is 1 year before opening.


Tungsten disulfide is a synthetic material with a wide range of electronic and optoelectronic applications. The diameter of a monolayer tungsten disulfide is 0.1~4um, which can be used to develop next-generation transistors, wearable electronic products, and even flexible biomedical equipment.
Since the first two-dimensional material graphene was discovered in experiments, people have been studying two-dimensional electronic materials for decades. Graphene and similar two-dimensional materials are only one atom thick, and are also called single-layer materials. Their thickness is hundreds of thousands of times smaller than the thickness of a piece of paper. These two-dimensional materials have huge advantages and potentials, namely high flexibility, strength and conductivity, but it is still very challenging to use them in practice.
Graphene (single layer of carbon atoms) has been studied for a long time in the field of transistors, but its lack of energy band gap has brought difficulties for semiconductor applications. Unlike graphene, tungsten disulfide has a considerable energy band gap, and it also shows new properties: when the number of atomic layers increases, the energy band gap becomes adjustable, and when it is at the thickness of a single atomic layer, it can strongly absorb and emit light, making it an ideal choice in the fields of optoelectronics, sensing and flexible electronic devices.


Tungsten trisulfide (WS3) is a reddish brown powder. Its solubility in water depends on temperature. It is soluble in alkaline solution; Dissolve in the solution of sodium sulfide and iron sulfide to form thiotungstate; Decomposition into tungsten disulfide and sulfur above 170 ° C; It can be reduced to metal tungsten by hydrogen, and can form metal tungsten when heated with lime.
Preparation of Tungsten Trisulfide (WS3)
The acidified ammonium tungstate solution is introduced with hydrogen sulfide to precipitate tungsten trisulfide.
